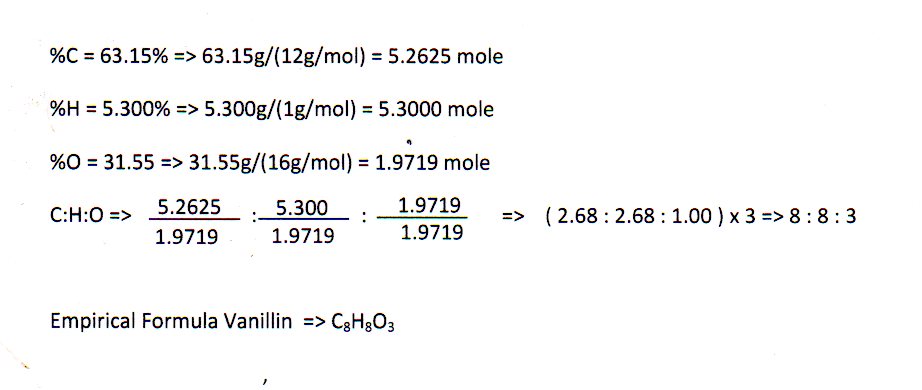
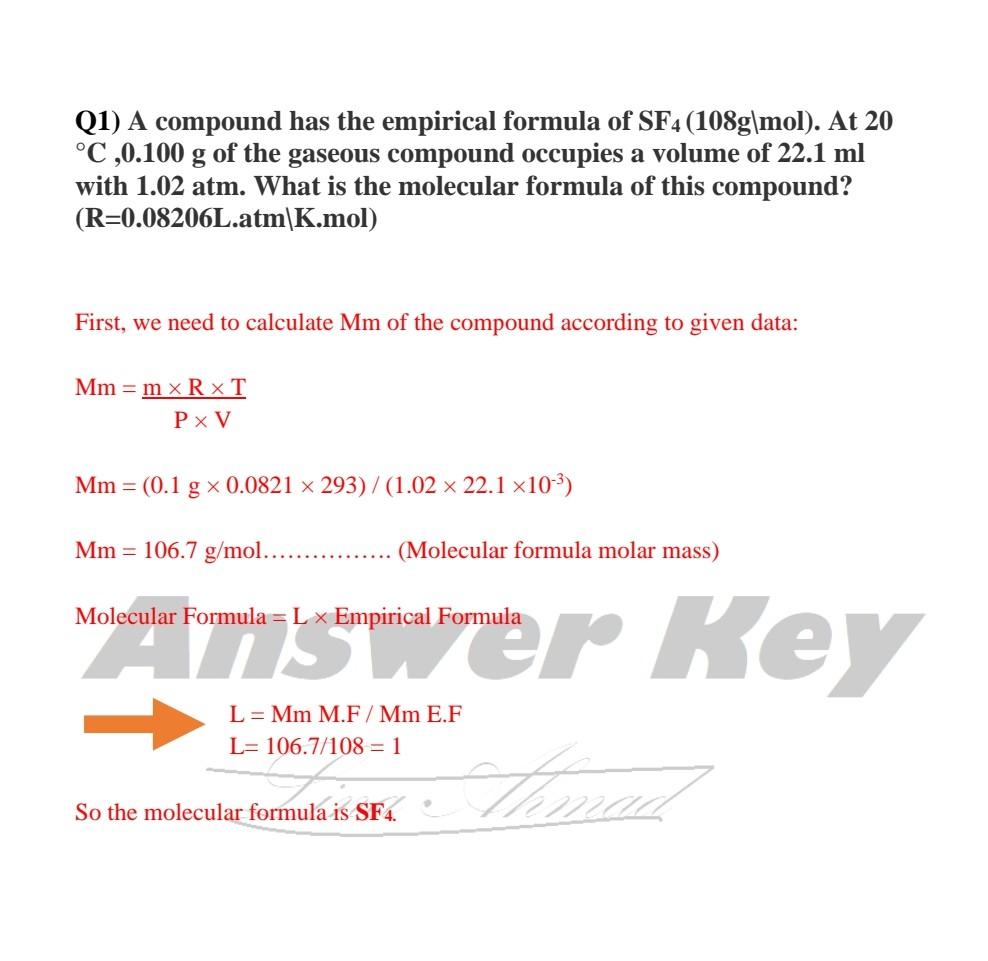
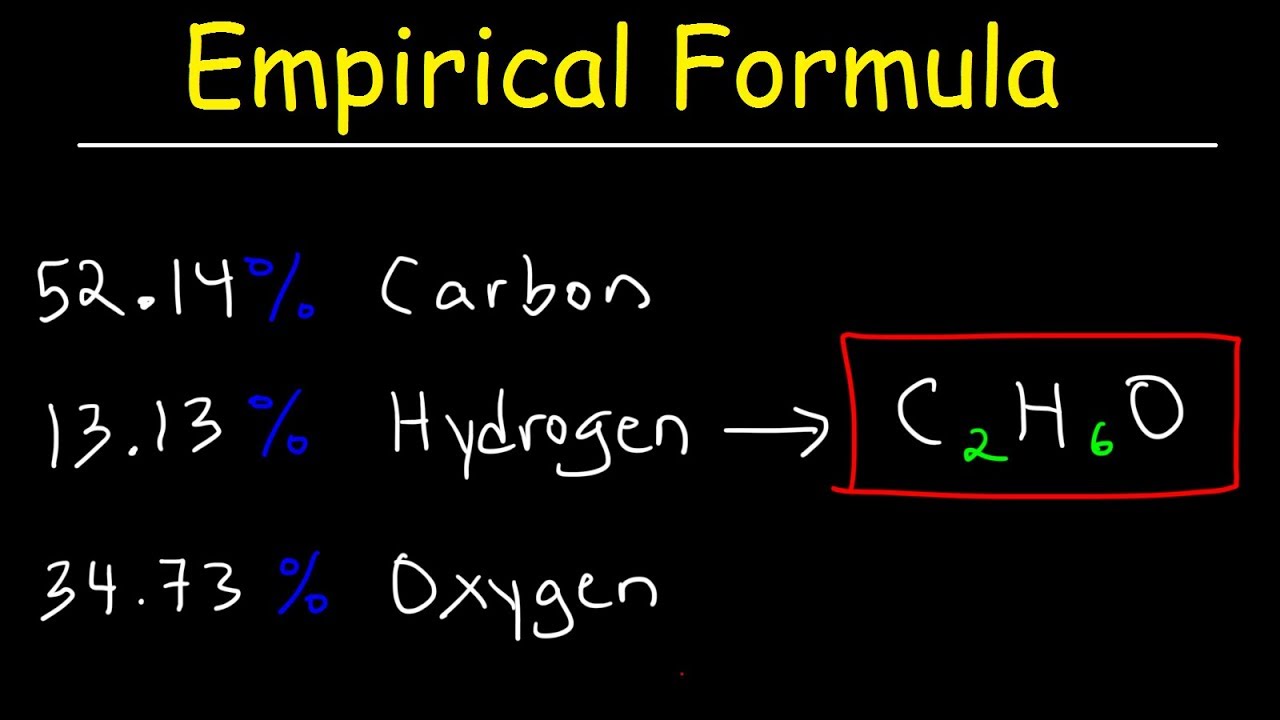
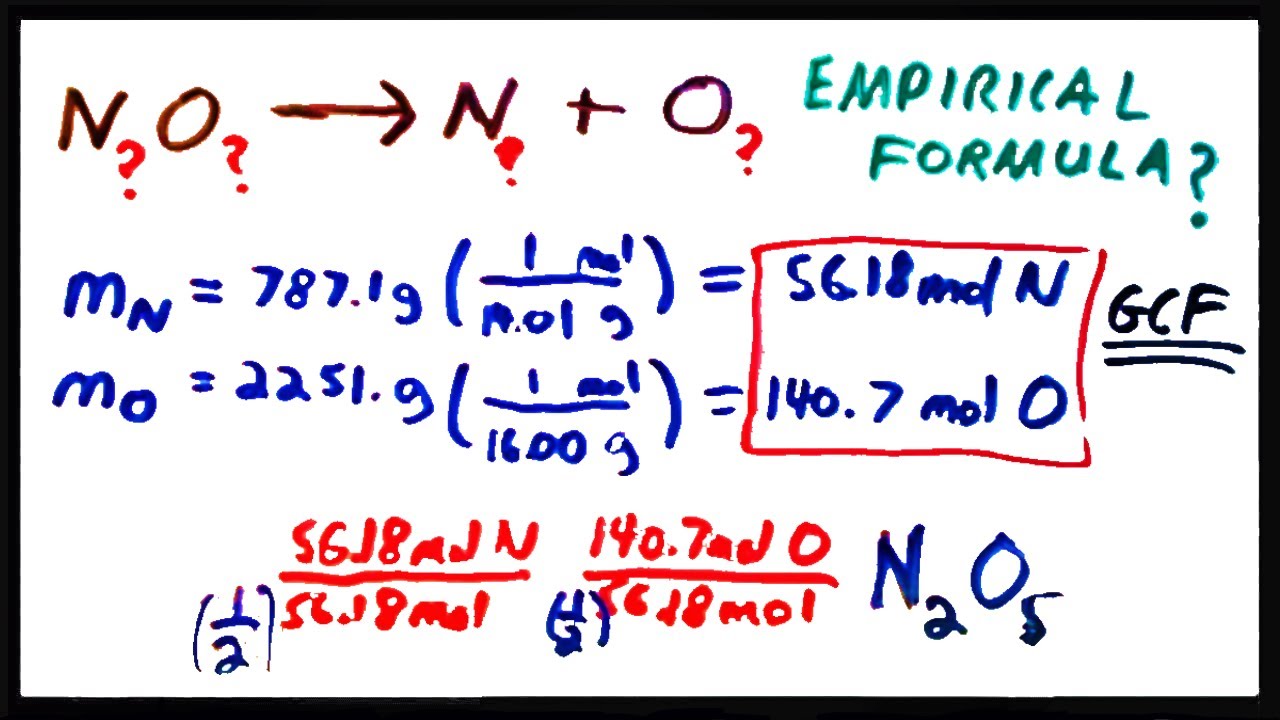
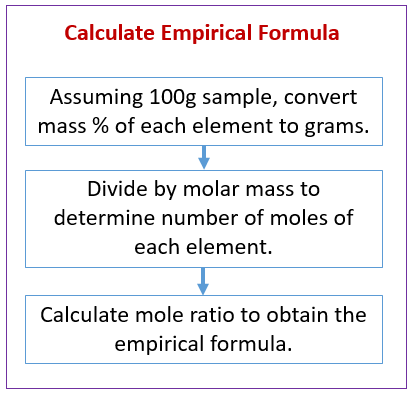
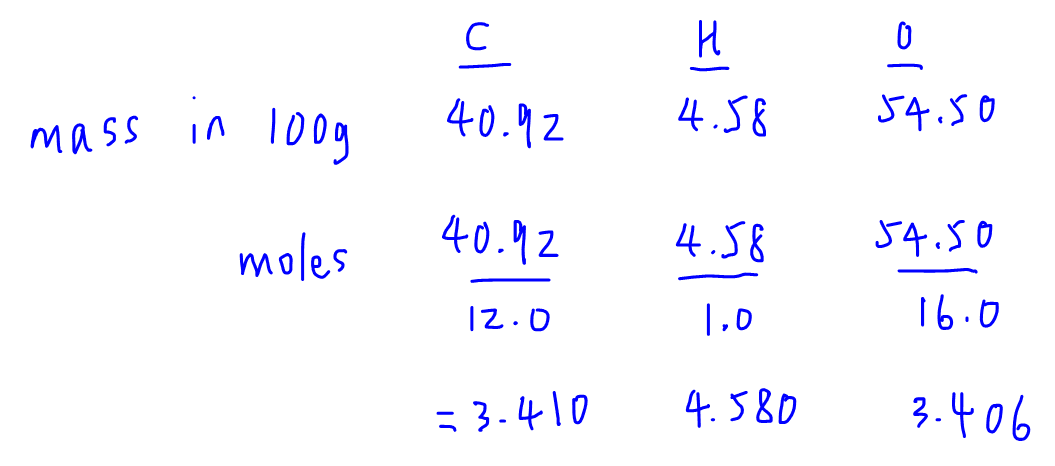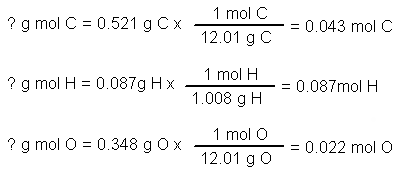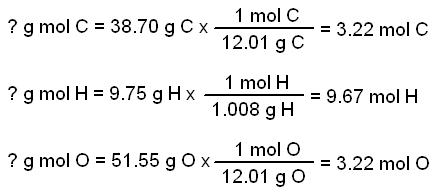A chemical compound is found to have the following composition : C = 19.5%, Fe = 15.2%, N = 22.8%, K = 42.5% Calculate the empirical formula of the compound. What will
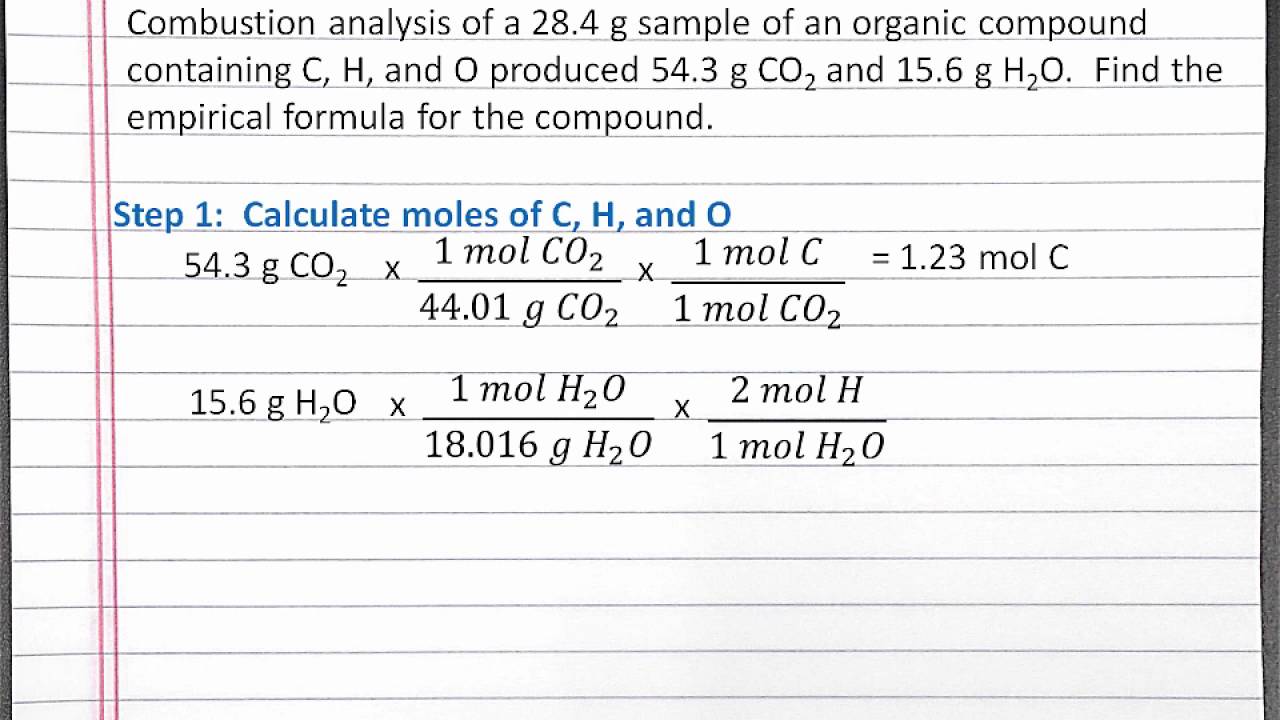
CHEMISTRY 101: Finding Empirical Formula Using Combustion Analysis for a Compound with C, H, O - YouTube
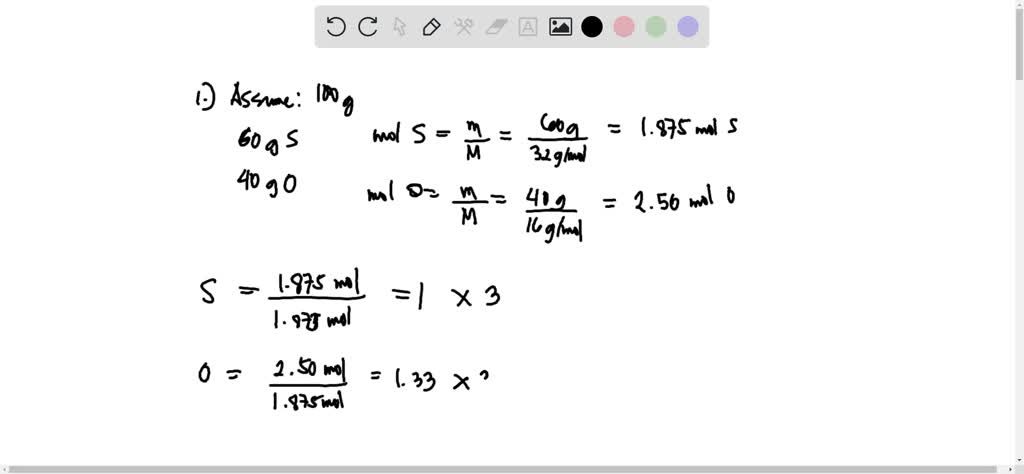
SOLVED: - What is the empirical formula of a compound containing 60.0% sulfur and 40.0% oxygen by mass? - Determine the molecular formula of the compound with an empirical formula of CH

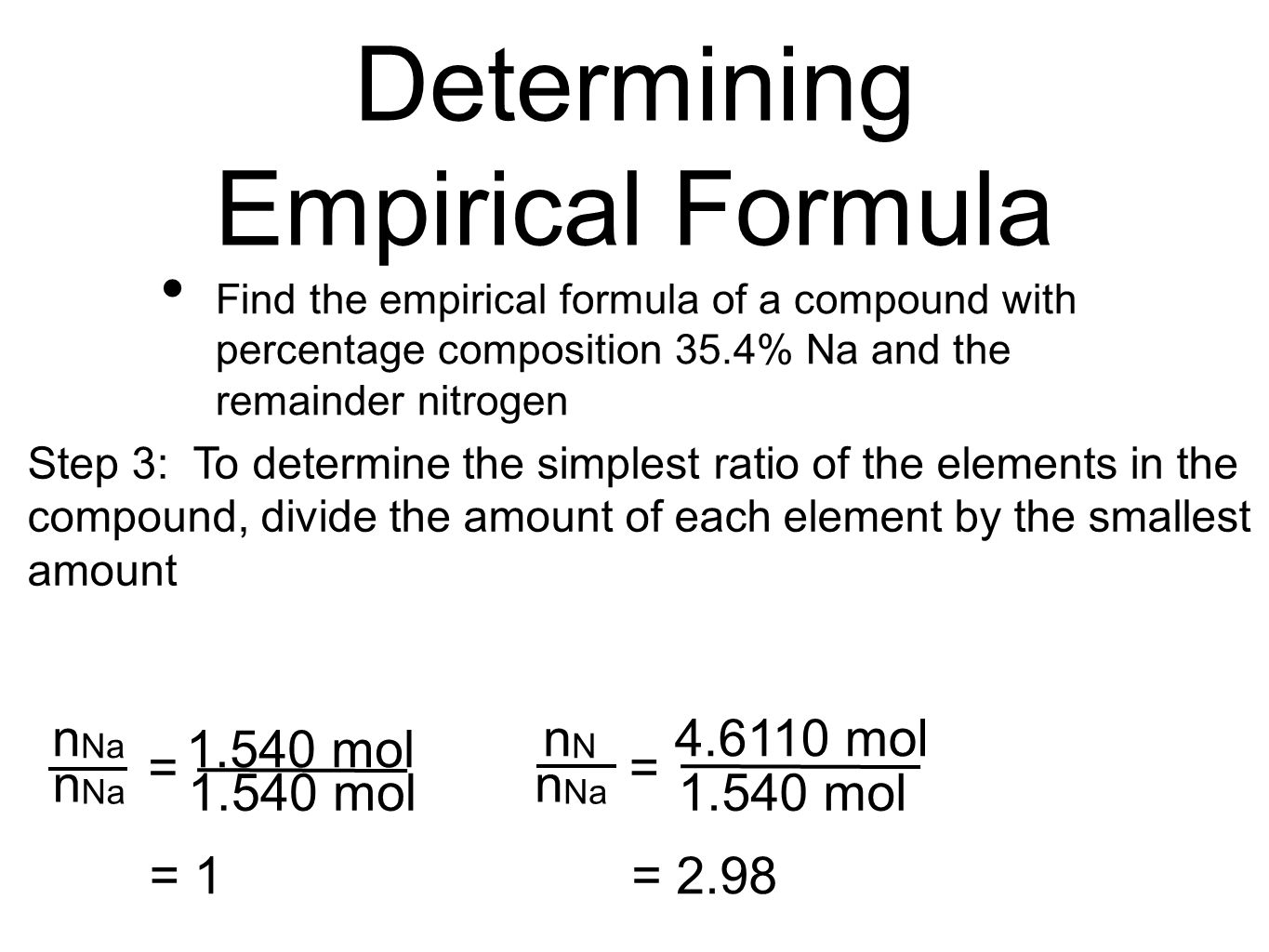


.PNG)