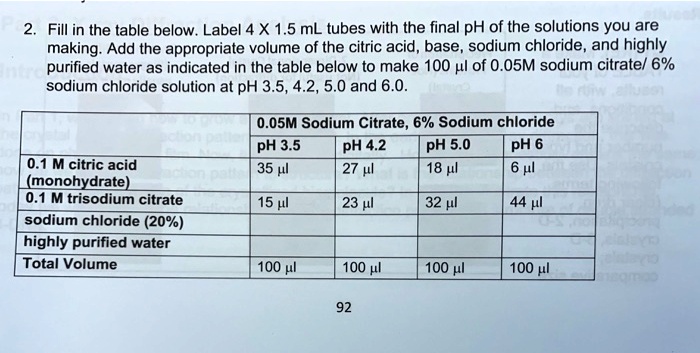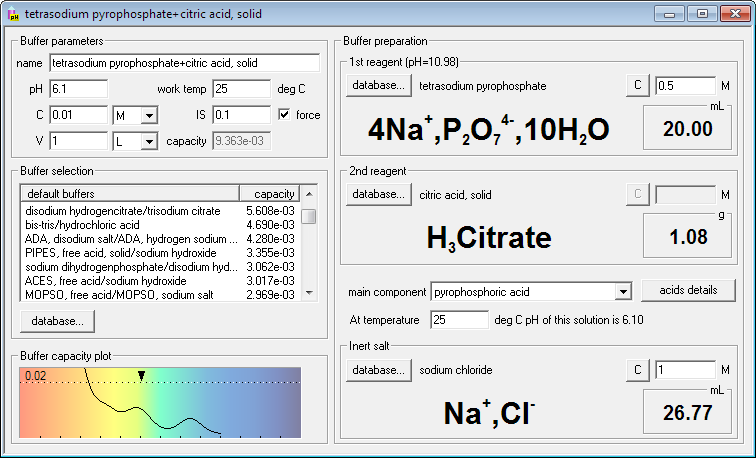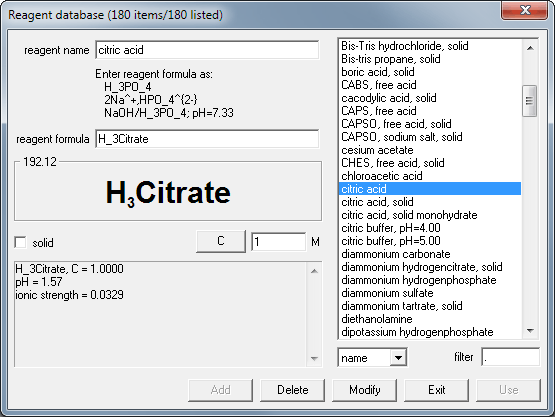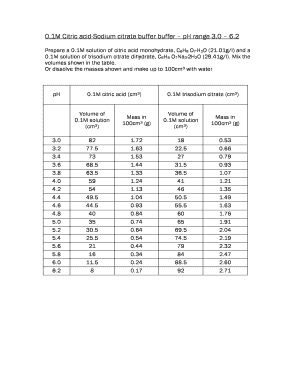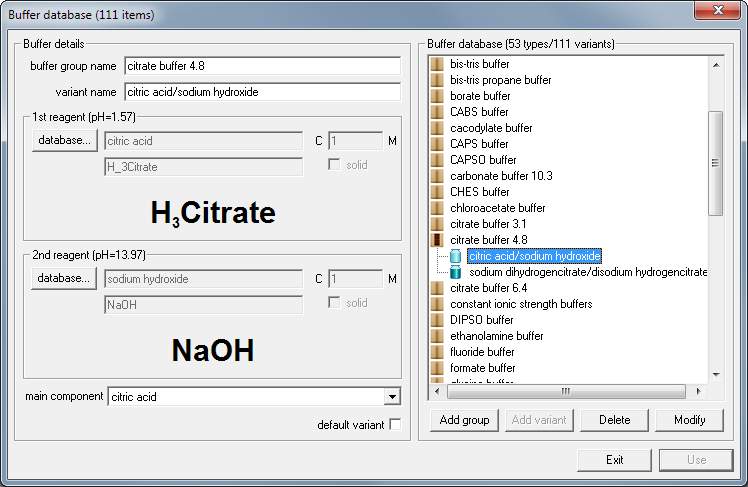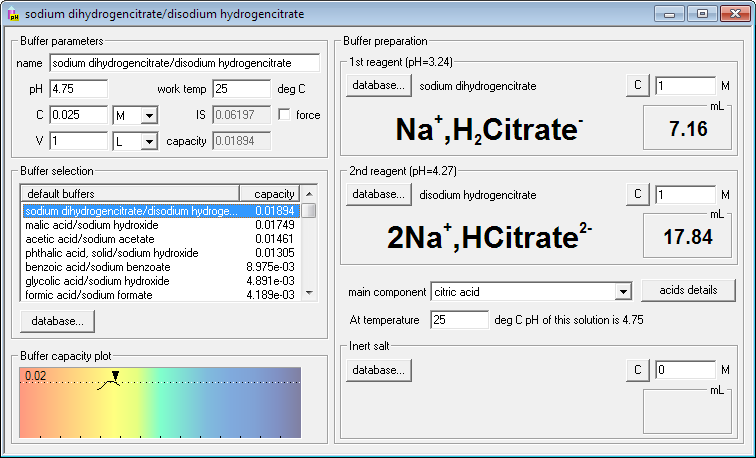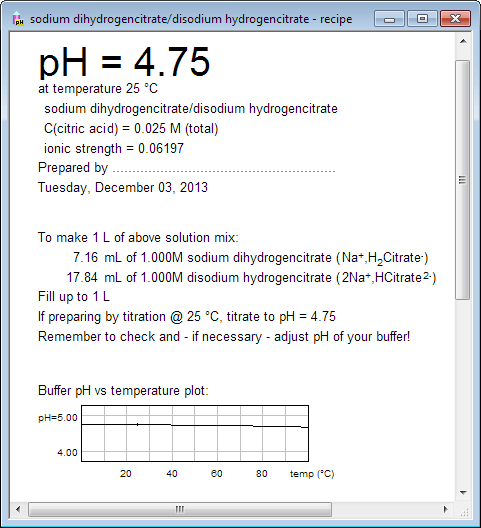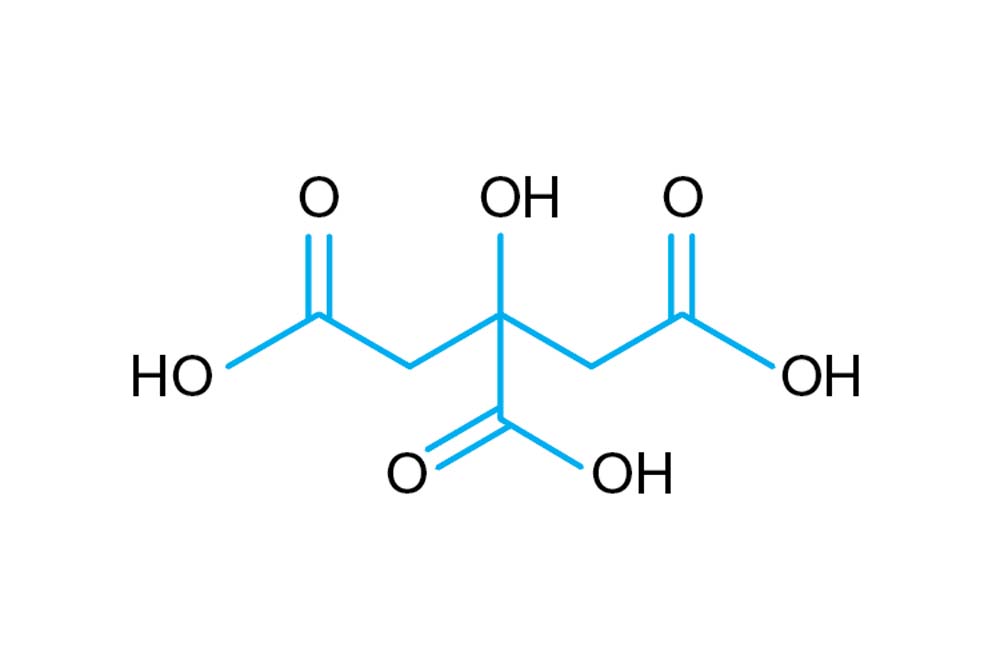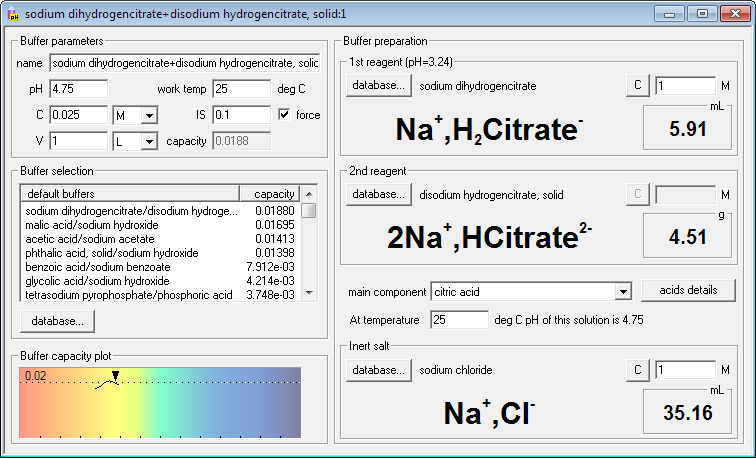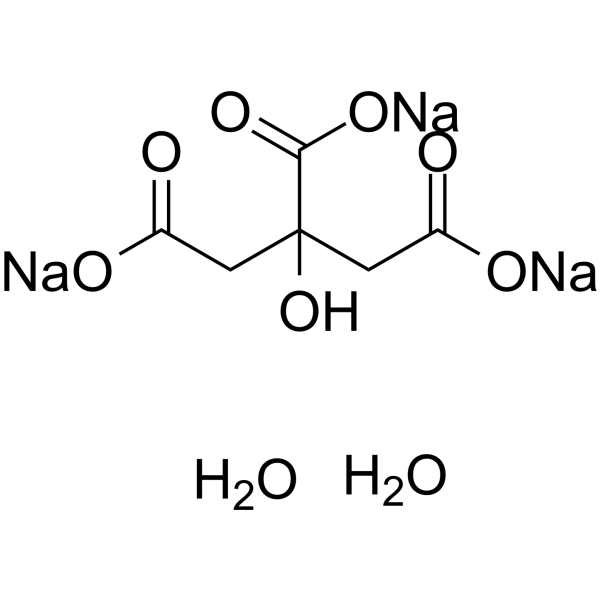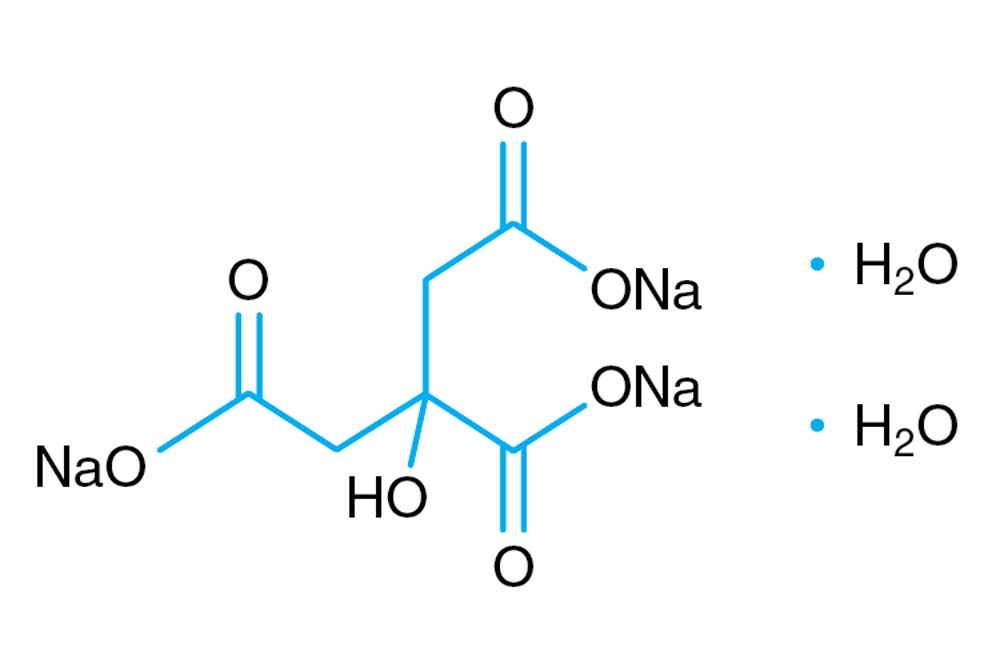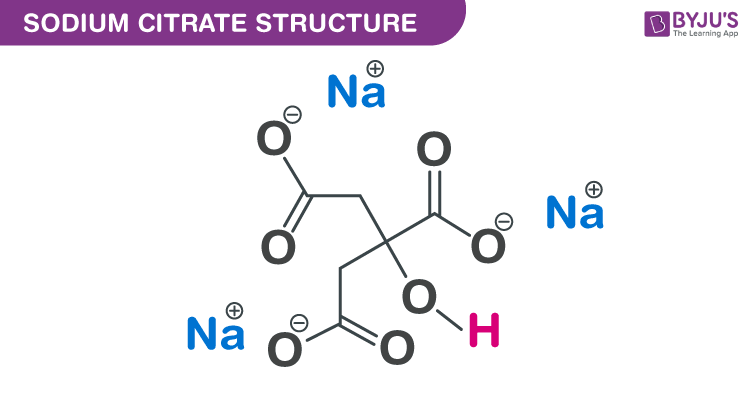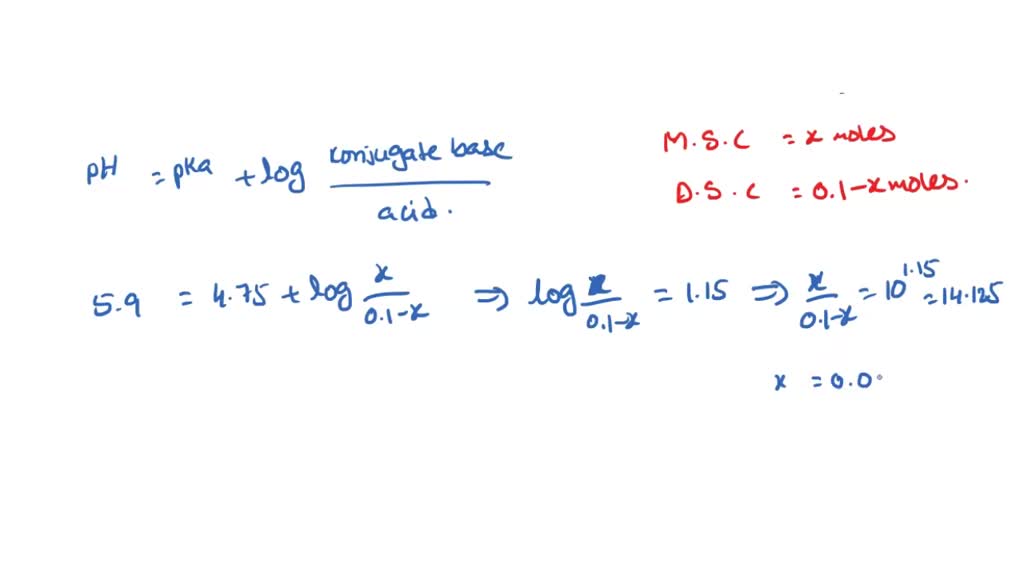
SOLVED: Describe the preparation of 1.0 L of 0.1 M citrate buffer, pH 5.90, starting with crystalline citric acid (FW = 210; pKa1 = 3.1; pKa2 = 4.75; pKa3 = 5.4) and 1.0 M NaOH.
![pH 4.25 Sodium Citrate Buffer Solution・199-07185[Detail Information] | [Analytical Chemistry]|Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation pH 4.25 Sodium Citrate Buffer Solution・199-07185[Detail Information] | [Analytical Chemistry]|Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation](https://labchem-wako.fujifilm.com/sc/06/199-07185.jpg)
pH 4.25 Sodium Citrate Buffer Solution・199-07185[Detail Information] | [Analytical Chemistry]|Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation